Overview |
| Name | Calcium |
| Symbol | Ca |
| Atomic Number | 20 |
| Atomic Weight | 40.078 |
| Density | 1.55 g/cm3 |
| Melting Point | 842 °C |
| Boiling Point | 1484 °C |
Thermal properties |
| Phase | Solid |
| Melting Point | 842 °C |
| Boiling Point | 1484 °C |
| Absolute Melting Point | 1115 K |
| Absolute Boiling Point | 1757 K |
| Critical Pressure | N/A |
| Critical Temperature | N/A |
| Heat of Fusion | 8.54 kJ/mol |
| Heat of Vaporization | 155 kJ/mol |
| Specific Heat | 631 J/(kg K)[note] |
| Adiabatic Index | N/A |
| Neel Point | N/A |
| Thermal Conductivity | 200 W/(m K) |
| Thermal Expansion | 0.0000223 K-1 |
Bulk physical properties |
| Density | 1.55 g/cm3 |
| Density (Liquid) | 1.378 g/cm3 |
| Molar Volume | 0.000025857 |
| Brinell Hardness | 167 MPa |
| Mohs Hardness | 1.75 |
| Vickers Hardness | N/A |
| Bulk Modulus | 17 GPa |
| Shear Modulus | 7.4 GPa |
| Young Modulus | 20 GPa |
| Poisson Ratio | 0.31 |
| Refractive Index | N/A |
| Speed of Sound | 3810 m/s |
| Thermal Conductivity | 200 W/(m K) |
| Thermal Expansion | 0.0000223 K-1 |
Reactivity |
| Valence | 2 |
| Electronegativity | 1 |
| ElectronAffinity | 2.37 kJ/mol |
| Ionization Energies | | 589.8, 1145.4, 4912.4, 6491, 8153, 10496, 12270, 14206, 18191, 20385, 57110 kJ/mol |
|
Health and Safety |
| DOT Hazard Class | |
| DOT Numbers | 1855 |
| RTECS Number | N/A |
| NFPA Label | 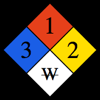 |
|
Classifications |
| Alternate Names | None |
| Names of Allotropes | None |
| Block | s |
| Group | 2 |
| Period | 4 |
| Series | Alkaline Earth Metal |
| Electron Configuration | [Ar]4s2 |
| Color | Silver |
| Discovery | | 1808 in The United Kingdom |
|
| Gas phase | N/A |
| CAS Number | CAS7440-70-2 |
| CID Number | |
| RTECS Number | N/A |
Electrical properties |
| Electrical Type | Conductor |
| Electrical Conductivity | 2.9×107 S/m |
| Resistivity | 3.399999999999×10-8 m Ω |
| Superconducting Point | N/A |
Magnetic properties |
| Magnetic Type | Paramagnetic |
| Curie Point | N/A |
| Mass Magnetic Susceptibility | 1.38×10-8 m3/Kg |
| Molar Magnetic Susceptibility | 5.531×10-10 m3/mol |
| Volume Magnetic Susceptibility | 0.00002139 |
Abundances |
| % in Universe | 0.007% |
| % in Sun | 0.007% |
| % in Meteorites | 1.1% |
| % in Earth's Crust | 5% |
| % in Oceans | 0.00042% |
| % in Humans | 1.4% |
Atomic dimensions and structure |
| Atomic Radius | 194 pm |
| Covalent Radius | 176 pm |
| Van der Waals Radius | N/A |
| Crystal Structure | Face-centered Cubic |
| Lattice Angles | |
| Lattice Constants | | 558.84, 558.84, 558.84 pm |
|
| Space Group Name | Fm_
3m |
| Space Group Number | 225 |
Nuclear Properties |
| Half-Life | Stable |
| Lifetime | Stable |
| Decay Mode | N/A |
| Quantum Numbers | 1S0 |
| Neutron Cross Section | 0.43 |
| Neutron Mass Absorption | 0.00037 |
| Known Isotopes | | 34Ca, 35Ca, 36Ca, 37Ca, 38Ca, 39Ca, 40Ca, 41Ca, 42Ca, 43Ca, 44Ca, 45Ca, 46Ca, 47Ca, 48Ca, 49Ca, 50Ca, 51Ca, 52Ca, 53Ca, 54Ca, 55Ca, 56Ca, 57Ca |
|
| Stable Isotopes | |
| Isotopic Abundances | |
|